M-Gard® is an immune booster that strengthens your body’s essential protective systems against various pathogens, including bacteria, viruses, fungi, and more*. Research has demonstrated that M-Gard® exhibits immune-regulating properties both in laboratory settings and in live experiments involving animal and human subjects. M-Gard® serves as an outstanding immune support supplement for human consumption.
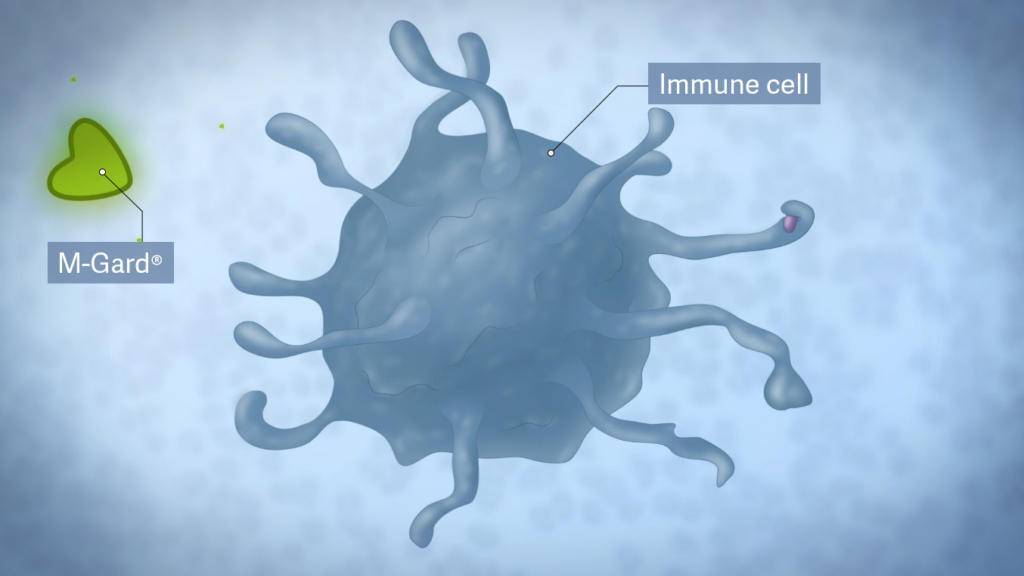
The unique molecular structure of the bioactive M-Gard® lets it bind to specific receptors on immune cells. This interaction is known to occur through different cell surface receptors — most notably, the Dectin 1 receptor.
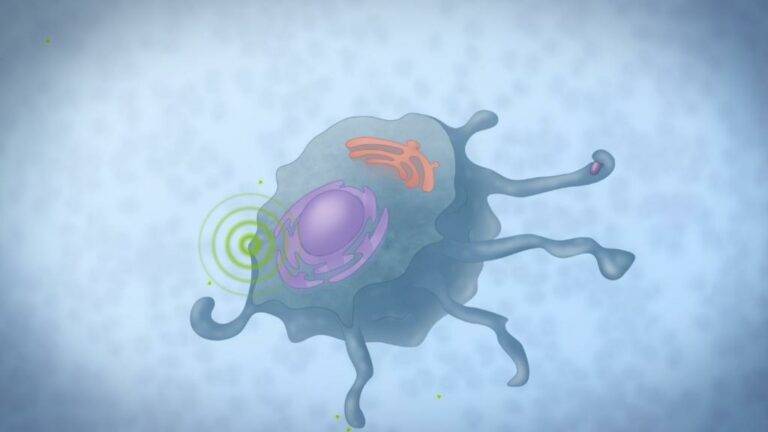
Binding of M-Gard® to a receptor triggers cellular signalling cascades, which modulates the immune cell that are part of the innate immune system.
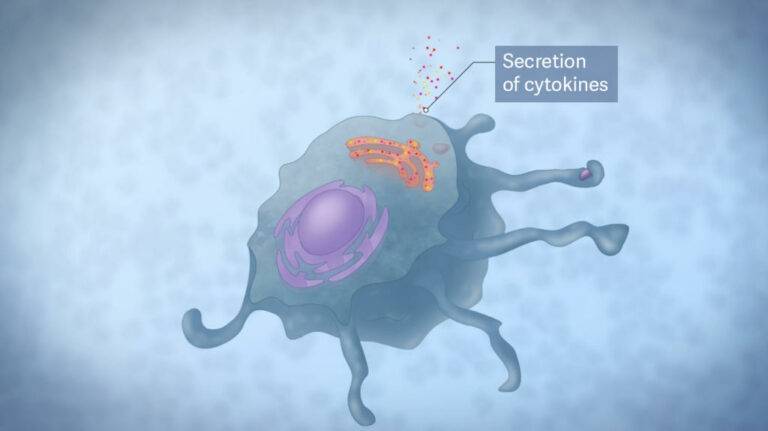
M-Gard® modulates the innate immune system, leading to enhanced immune responses (increased cytokine secretion) during infection. The innate immune system is the first-line defense against infections and controls the responses of the specific immune system.
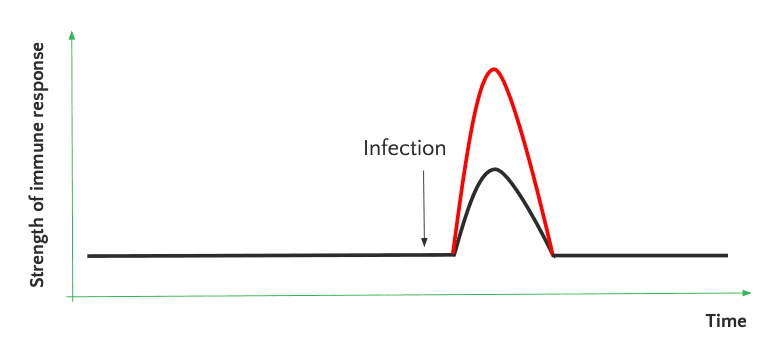
Trained immunity induces enhanced immune properties in innate immune cells resulting in an increased immune response to subsequent infections and better health.

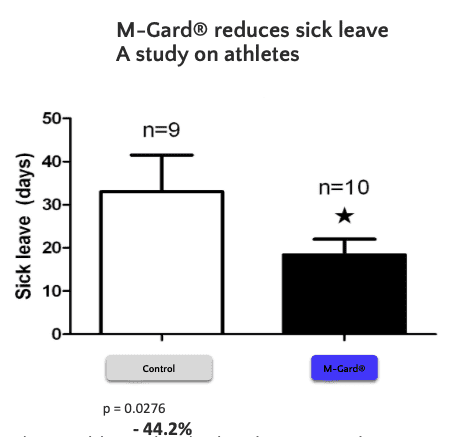
One-year study. Healthy individuals taking/not taking M-Gard® dietary supplement.
Number of sick leave days related to symptoms like cold, flu and fever.
70 subjects per treatment group. Total number of subjects was 140.
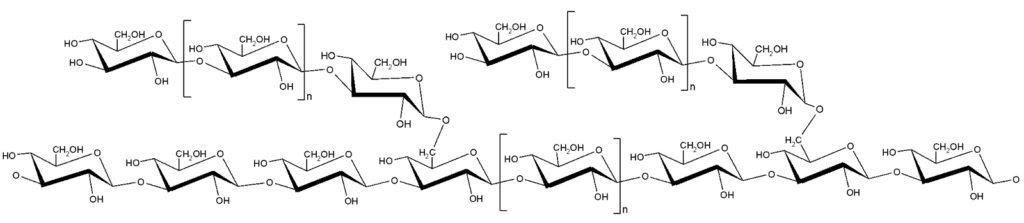
M-Gard® (beta-1,3/1,6-glucan) 720 mg (two capsules), once daily, orally
Cellulose (two capsules), once daily, orally
8 weeks
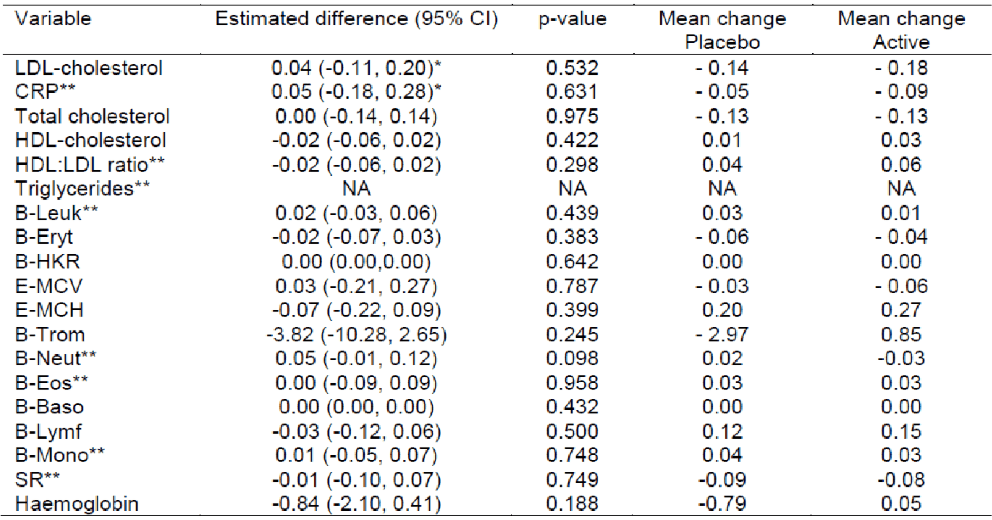
A critical review of the adverse events of the study gave no reason to believe that there were any safety concerns associated with M-Gard®.
No significant difference in the effect of placebo and M-Gard® were seen on LDL-cholesterol. Since no values of immunological variables were above the cut-off limit, M-Gard® does apparently not induce any response leading to high cytokine levels.
Three groups, each with six individuals, received either 100mg/day (group 1), 200mg/day (group 2) or 400 mg/day (group 3).
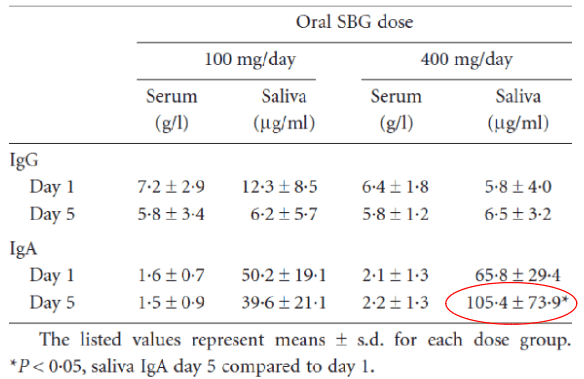
Repeated measurements of beta-glucan in serum revealed no systemic absorption of the agent following the oral doses of SBG. In saliva, the immunoglobulin A concentration increased significantly for the highest SBG dose employed. SBG was thus safe and well tolerated by healthy volunteers, when given orally once daily for 4 consecutive days at doses up to 400 mg.
Trained immunity induces enhanced immune properties in innate immune cells resulting in an increased immune response to subsequent infections and better health.

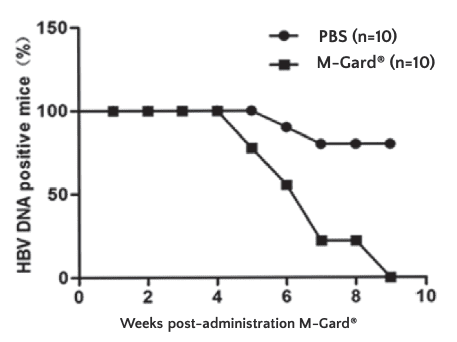
Mice given M-Gard® had 100% (10/10) clearance of HBV DNA
at 9 weeks post treatment. While only 30% (3/10) of mice in the control group had clearance of HBV DNA at the same time point.
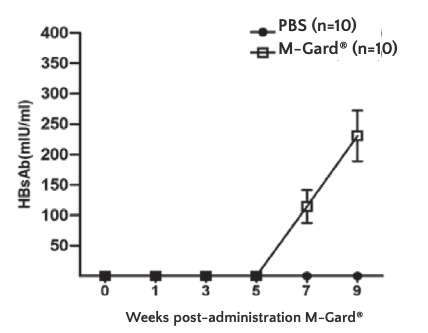
HBs antibodies were detectable at week 7 in mice given M-Gard®, demonstrating that M-Gard® induced specific immune responses against HBV.
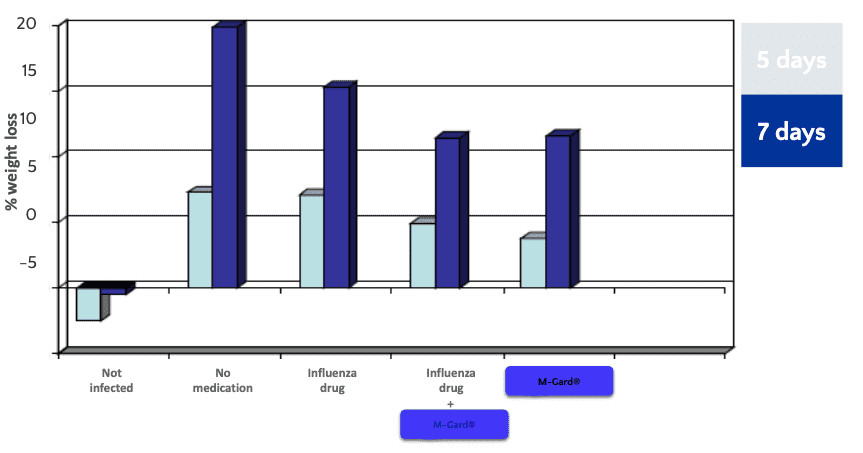
M-Gard® was given orally from two days prior to infection and daily until termination of experiment, and a prominent anti-influenza drug daily from day 1 after infection. Influenza-virus (A/PR8, H1N1) was administered into the lungs.
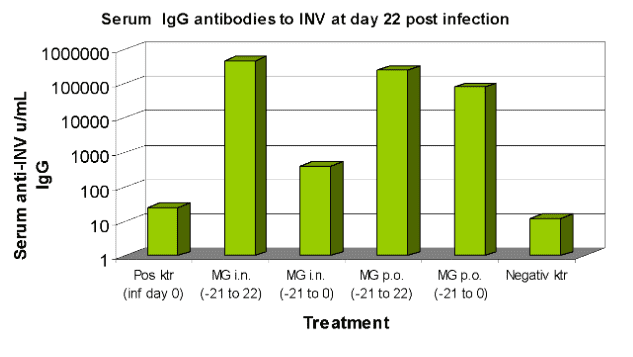
Mice given 0.1 mg M-Gard® either into the nasal cavity (i.n.) or orally (p.o.) once per day for 21 (up to day 0) or 43 (up to day 22) days.
After 21 days (day 0) the mice were infected with live influenza virus.
After 43 days (day 22 post infection) blood samples were collected and analysed for serum IgG-titer against the virus.
Negative control= not infected
Pigs are closely related to humans in terms of anatomy, genetics and physiology, and represent an excellent animal model to study various microbial infectious diseases.
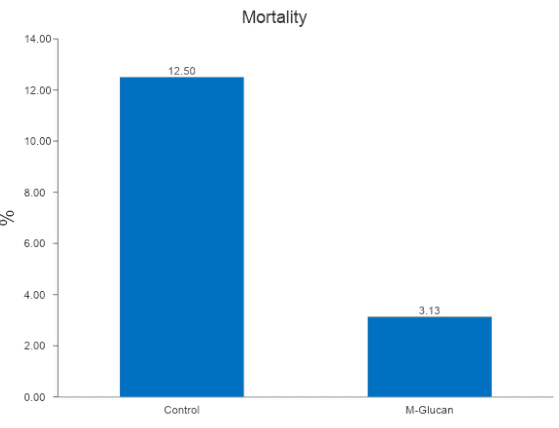
Mortality were extremely high in the first 7-21 days indicating the ‘dirty’ environment was a challenge to the piglets. No antibiotics or ZnO was fed.
M-Glucan fed piglets had lower mortality compared to control fed piglets.
Clinical trial program of the bivalent ganglioside vaccine in combination with SBG® for high-risk neuroblastoma.